PharmaCare Newsletter
April 2025 PharmaCare Newsletter
On this page:
Find past issues on the newsletter search page.

The current edition of PAD Refills is an update to BC Provincial Academic Detailing Service’s 2020 Table 1: Antidepressant Drug Information. Make sure to subscribe so you don’t miss out on news and updates!
Inflectra® discontinuation and biosimilar infliximab listings
Effective April 1, 2025, the Inflectra®-branded infliximab product will be discontinued. Starting that same day, Remdantry™ will be marketed by Celltrion Healthcare under the same Health Canada–assigned DIN (02419475), as it is the same product.
Between April 1 and September 30, 2025, Pfizer Canada ULC will continue to distribute Inflectra. During that period, pharmacies must dispense Inflectra under PIN 66128531 for it to be eligible for PharmaCare coverage.
As of October 1, 2025, Inflectra will no longer be a PharmaCare benefit.
Also on April 1, 2025, the infliximab biosimilar Ixifi® will be marketed by Pfizer Canada ULC under DIN 02523191.
Avsola® and Renflexis™ continue to be available as intravenous infliximab biosimilar options, and Remsima™SC continues to be available as a subcutaneous option.
Patient transition
April 1, 2025
As soon as it is discontinued on April 1, 2025, the Inflectra-branded product will not be covered for new patients, and Special Authority (SA) requests for infliximab will only be approved for Avsola, Ixifi, Remdantry, Remsima SC or Renflexis.
Existing patients who have been dispensed the Inflectra-branded product in the previous 3 months and have PharmaCare coverage for Inflectra, will have transitional coverage until September 30, 2025. This will allow time to switch to Avsola, Ixifi, Remdantry, Remsima SC or Renflexis.
April 1 to September 30, 2025
During this 6-month period, existing patients with PharmaCare coverage for Inflectra must switch to Avsola, Ixifi, Remdantry, Remsima SC or Renflexis.
October 1, 2025
Inflectra-branded product is no longer available and therefore not an eligible PharmaCare benefit. Only Avsola, Ixifi, Remdantry, Remsima SC and Renflexis are covered by PharmaCare.
| Inflectra discontinuation timeline | |||
|---|---|---|---|
| April 1, 2025 | April 1, 2025, to September 30, 2025 | October 1, 2025 | |
|
Inflectra discontinued, not covered for new patients. Existing patients have 6-month transitional coverage. Inflectra claims for existing patients must be submitted with PIN 66128531. |
Patients with PharmaCare coverage for Inflectra must switch to Avsola, Ixifi, Remdantry, Remsima SC or Renflexis to maintain coverage. | Inflectra becomes a PharmaCare non-benefit. | |
New Special Authority (SA) approval is only required when a patient’s existing coverage expires. SA approval is for all biosimilar infliximab formulations covered for the indication, not limited to the specific product selected on the SA form.
| Switching options: PharmaCare-covered infliximab products | |||
|---|---|---|---|
| Brand name | Strength & form | Limited coverage criteria | |
|
Avsola® |
100 mg/vial powder for solution (intravenous) | ||
| Remsima™SC | 120 mg/mL pre-filled pen for subcutaneous injection | ||
For patients transitioning from Inflectra to Remdantry
- A new prescription will not be required
- A different patient support program* is available
For patients transitioning from Inflectra to Ixifi
- A new prescription will be required
- The patient support program* remains the same
For patients transitioning from Inflectra to Avsola, Renflexis or Remsima SC
- A new prescription will be required
- A different patient support program* is available
*PharmaCare coverage is not tied to participation in a patient support program.
For a list of patients currently receiving Inflectra, prescribers can contact a Pfizer representative from the Inflectra patient support program by phoning 1-855-935-3539 (Monday to Friday, 8 am to 8 pm EST) or by email at inflectra@pfizerflex.com
Patient support program contact information
| Avsola® | Amgen SupportPlus Tel: 1-877-936-2735 Fax: 1-888-987-2201 Email: info@oneenliven.ca Website: www.amgen.ca/en-CA/about/contact-us |
|||
|---|---|---|---|---|
| Ixifi® | PfizerFlex Tel: 1-800-935-3539 Website: https://www.pfizerflex.ca/ |
|||
| Remdantry™ | Celltrion Connect Tel: 1-855-966-1648 Email: support@celltrionconnect.ca Website: https://www.celltrionhealthcare.ca/contactus/connect/ |
|||
| Remsima™ SC | ||||
| Renflexis™ | Harmony by Organon Tel: 1-866-556-5663 Email: info@harmonybyorganon.ca Website: https://harmonyorganon.ca/ |
|||
For questions or concerns, please contact the Formulary Management team by email at FM.GenericMailbox@gov.bc.ca
Three-year renewal option for plaque psoriasis biologics
Beginning April 1, 2025, a Special Authority coverage renewal period of three years will be available for biologic agents treating plaque psoriasis.
This means that dermatologists will be able to choose either one year or three years of Special Authority coverage when renewing coverage for patients using biologics for plaque psoriasis.
Dermatologists on the Psoriasis Drug Benefit Adjudication Advisory Committee (PDBAAC) agree that the three-year renewal option will reduce workload for specialists without compromising patient care. This supports the work of the Administrative Burdens Working Group, a partnership between the Ministry of Health, Doctors of BC, and Health Quality BC.
This extended coverage duration may also result in improved patient access to these medications as there will be fewer chances for gaps in coverage to occur. This is especially important for patients in rural or remote areas, or those with barriers for regular reassessment.

The 3-year Special Authority coverage renewal option is available for all eligible strengths and formulations of the biologics covered by PharmaCare for plaque psoriasis:
- Adalimumab biosimilars
- Bimekizumab
- Etanercept biosimilars
- Infliximab biosimilars
- Ixekizumab
- Risankizumab
- Secukinumab
- Ustekinumab biosimilars
FNHA transitioning select benefits from PBC to Plan W
The First Nations Health Authority (FNHA) is transitioning coverage for some drug therapies on the FNHA-Pacific Blue Cross (PBC) Supplemental Formulary to Plan W. The transition is to simplify drug coverage processes for pharmacy providers and FNHA clients.
To ensure continuity of care, FNHA clients currently using medications on the Supplemental Formulary that are not being added to Plan W will be provided ongoing coverage through PharmaCare. In the case of any unexpected legacy coverage issues, pharmacists can dispense a one-time fill of a drug to the client at no cost and use FNHA’s Transitional Payment Request process to be reimbursed the cost of the claim plus $10.
Stay up to date with changes to the Supplementary Formulary over the coming months by referring to the FNHA-PBC Pharmacy Fee Supplement (PDF, 1.4MB). For the most current list of DINs covered under Plan W, visit the PharmaCare Formulary Search and Plan W OTC drug list web page.
Further updates will also be announced in the PharmaCare Newsletter. Make sure you subscribe for notifications of new editions.
For any client-specific questions or concerns, pharmacy teams can contact FNHA Health Benefits at 1-855-550-5454 (ext. 4 then ext. 2).
Resources
- Plan W – Information for health professionals
- FNHA Transitional Payment Request
- FNHA-PBC Pharmacy Fee Supplement (PDF, 1.4MB)
- Plan W OTC drug list
Medical Laboratory Week – April 13-19
Medical Laboratory Week is celebrated April 13 –19 to recognize laboratory professionals across the province and their critical role in the healthcare system.
The year 2025 marks 40 years of Canada celebrating Medical Laboratory Week and is the fourth year that the Province of B.C. has formally recognized the celebration with a Proclamation (PDF, 857KB).
This year’s theme is “medical laboratory professionals illuminate the path to diagnosis.”
The work of laboratory professionals is critical in supporting other healthcare professionals with making accurate diagnoses. Laboratory results inform over 70% of clinical decisions.
Medical laboratory professionals include:
- Medical laboratory assistants and technologists
- Combined laboratory x-ray technologists
- Diagnostic cytology technologists
- Clinical genetics technologists
- Laboratory medicine physicians and pathologists
- Laboratory administrators
Their behind-the scenes work analyzing blood, tissues, and other samples is paramount to supporting patient care.

Take a moment during Medical Laboratory week to to thank medical laboratory professionals for their hard work and dedication! Visit National Medical Laboratory Week and Medical Laboratory Professionals Week for a schedule of the week’s events and to learn more.
Resources
- 2025 Proclamations
- National Medical Laboratory Week – Canadian Society for Medical Laboratory Science
- Medical Laboratory Professionals Week – British Columbia Society for Medical Laboratory Science
Drug shortage: Nicorette® lozenges
Due to manufacturing disruptions, Nicorette is experiencing a shortage of 4 mg mint prescription lozenges (PIN: 80112095).
As a mitigation measure, PharmaCare is temporarily covering
over-the-counter (OTC) packaged Nicorette 4 mg mint lozenges
(PIN: 80053100).
Pharmacies can bill for the OTC Nicorette product using the prescription product PIN (80112095) for full reimbursement (up to $38.80 for 80 lozenges). The shortage is expected to resolve in April 2025.
Resources
Ostomy supplies PINs updated on March 24, 2025
Effective March 24, 2025, PharmaCare added more examples of eligible items and removed discontinued products from the Ostomy Supplies PINs list. New PINs were also created for items that were already covered under other categories on the list. If you dispense ostomy supplies, please refer to the updated list and ensure you start using these new PharmaCare PINs where appropriate.
Ostomy supplies are covered for eligible patients who have undergone bowel and/or bladder surgery that results in a colostomy, ileostomy, or urostomy, requiring an external pouch. Actual reimbursement is subject to the rules of the patient’s PharmaCare plan, including any Fair PharmaCare deductible requirements.
PharmaCare covers certain ostomy supplies for First Nations Health Authority clients under Plan W. FNHA provides additional items through their medical supply and equipment benefits program (refer to FNHA-PBC Pharmacy Fee Supplement (PDF, 1.4MB)).
Resources
Policy spotlight: Trial Prescription Program
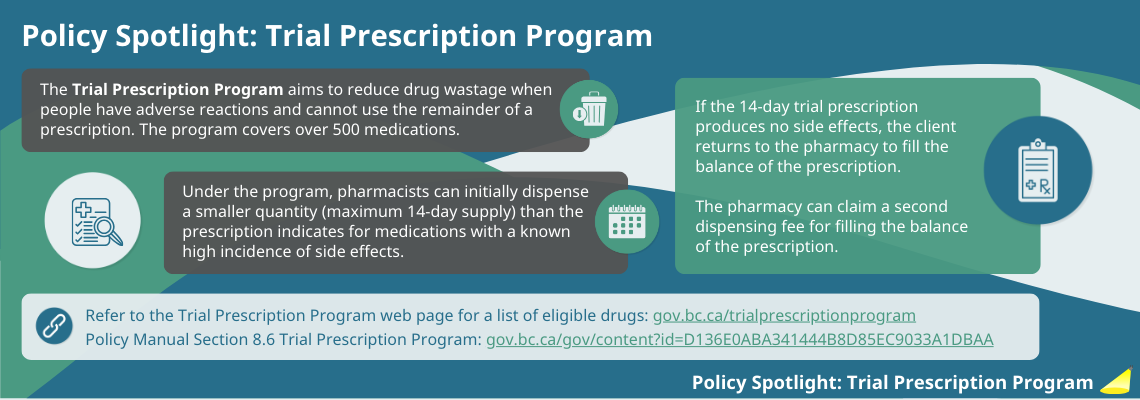
Resources
- Trial Prescription Program eligible products
- PharmaCare Policy Manual, Section 8.6: Trial Prescription Program
Limited coverage benefits: Infliximab (Ixifi®, Remdantry™)
PharmaCare has added the following limited coverage items to the PharmaCare drug list. Special Authority approval is required for coverage.
| Drug name | infliximab (Ixifi®) | ||
|---|---|---|---|
| Date effective | April 1, 2025 | ||
| Indication | For the treatment of moderately to severely active Crohn’s disease; psoriatic arthritis; ankylosing spondylitis; moderate to severe plaque psoriasis; rheumatoid arthritis; and moderate to severe ulcerative colitis. | ||
| DIN | 02523191 | Strength & form | 100 mg/vial powder for solution (intravenous) |
| Drug name | infliximab (Remdantry™) | ||
|---|---|---|---|
| Date effective | April 1, 2025 | ||
| Indication | For the treatment of moderately to severely active Crohn’s disease; psoriatic arthritis; ankylosing spondylitis; moderate to severe plaque psoriasis; rheumatoid arthritis; and moderate to severe ulcerative colitis. | ||
| DIN | 02419475 | Strength & form | 100 mg/vial powder for solution (intravenous) |
| Special notes | Remdantry has the same DIN as Inflectra, which will be discontinued in Canada on September 30, 2025. Refer to Inflectra® discontinuation and new biosimilar infliximab listings article above for more details. | ||
Criteria amendment: Sacubitril/valsartan (Entresto™)
PharmaCare has amended the coverage criteria for the following item.
| Drug name | sacubitril/valsartan (Entresto™) | ||
|---|---|---|---|
| Date effective | March 25, 2025 | ||
| Indication | For the treatment of heart failure (HF) with reduced ejection fraction in patients with New York Heart Association (NYHA) class II or III HF. | ||
| DINs | 02446928 02446944 02446936 |
Strength & form | 24 mg/26 mg tablet 49 mg/51 mg tablet 97 mg/103 mg tablet |
| Special notes | Limited coverage criteria for sacubitril/valsartan has been amended to permit prescribers who are experienced with the treatment of heart failure to apply for Special Authority coverage for sacubitril/valsartan. Please refer to the sacubitril/valsartan limited coverage criteria for additional information. | ||
EDRD coverage: Palovarotene (Sohonos®)
| Drug name | Palovarotene (Sohonos®) | ||
|---|---|---|---|
| Date effective | March 20, 2025 | ||
| Indication | To reduce the formation of heterotopic ossification in female patients aged 8 years and older and male patients aged 10 years and older with fibrodysplasia ossificans progressive. | ||
| DINs | 02524627 02524635 02524643 02524651 02524678 |
Strength & form | 1 mg oral capsule 1.5 mg oral capsule 2.5 mg oral capsule 5 mg oral capsule 10 mg oral capsule |
On March 20, 2025, the Ministry of Health initiated funding of palovarotene (Sohonos®) through PharmaCare’s Expensive Drugs for Rare Diseases (EDRD) process. Clinicians may apply for funding through this process for eligible patients with fibrodysplasia ossificans progressive. Palovarotene will be available through Bayshore pharmacy.
Discontinuation: Hypurin (porcine insulin)
Wockhardt UK, the sole supplier of animal-sourced insulin in Canada, has decided to discontinue the sale and distribution of Hypurin Regular Pork Insulin and Hypurin NPH in Canada. Canada’s inventory of Hypurin Regular Pork Insulin expires in December 2025 and Hypurin NPH in April 2026.
To extend supply, Health Canada authorized the importation of UK-authorized Hypurin Regular Pork Insulin and Hypurin NPH vials, which expire in May 2026. No additional inventory will be available in Canada after existing supply has been exhausted.
Hypurin Regular Pork Insulin and Hypurin NPH are the last animal-based insulins on the Canadian market. Biosynthetic human insulin and insulin analogues have been the standard treatment for insulin-dependent diabetes since the 1980s. Animal-derived insulins have not been available in other countries (such as the US and Australia) since the early 2000s.
Patients are encouraged to speak to their healthcare provider or pharmacist if they have questions or concerns about this discontinuation, and to discuss alternative treatments.
Resources
- Notice: Animal-sourced insulin discontinuation – Health Canada
- Hypurin_discontinuation_brief_- Canadian Society of Healthcare-Systems Pharmacy, Canadian Pharmacists Association, and The Canadian Society for Endocrinology and Metabolism (PDF, 172KB)
| Drug name | Form | DIN | Expiry of current supply | Expiry of UK–authorized supply | |
|---|---|---|---|---|---|
| Hypurin® Regular Pork Insulin | Vial – solution for injection | 02275872 | December 2025 | May 2026 | |
| Hypurin® NPH Insulin Isophane Pork | Vial – solution for injection | 02275864 | April 2026 | May 2026 | |
Your Voice: Input needed for drug decisions
The knowledge and experience of patients, caregivers and patient groups is integral to B.C.’s drug review process. If you know someone who is taking one of the drugs below or who has a condition any of the drugs treat, please encourage them to visit www.gov.bc.ca/BCyourvoice.
Your Voice is now accepting input on the following drugs:
| Drug | Indication | Input window | |
| semaglutide (Wegovy®) | Chronic weight management in adult patients with established cardiovascular disease | March 26 to April 22 at 11:59 pm | |
| elafibranor (TBC) | Primary biliary cholangitis (PBC) in adults | March 26 to April 22 at 11:59 pm | |

About the PharmaCare Newsletter
The PharmaCareNewsletter is published on the first Tuesday of each month (or Wednesday, if following a long weekend), with occasional mid-month special releases. The PharmaCare Newsletter communicates drug listings, PharmaCare policy, PharmaNet procedures, and other pertinent information for PharmaCare providers and health care partners.
Information in previous newsletters is accurate as of the date it was published. Newsletters are not retroactively updated when policy, procedures or other information changes. Refer to the most recent mention of a topic for up-to-date information.
Search past newsletters on the Newsletter search page.
Subscribe
Enter your email address to subscribe to updates of this page.
 The PharmaCare Newsletter team works from the territory of the Lekwungen People, including the Songhees and Esquimalt Nations. Our gratitude extends to them, and all the Indigenous Peoples on whose territories and lands we build relationships.
The PharmaCare Newsletter team works from the territory of the Lekwungen People, including the Songhees and Esquimalt Nations. Our gratitude extends to them, and all the Indigenous Peoples on whose territories and lands we build relationships.
BC PharmaCare counts on pharmacy and device providers to practise cultural safety and humility.
To learn more, read Coming Together for Wellness, a series of articles by First Nations Health Authority (FNHA) and PharmaCare, and consider taking the online San’yas Indigenous Cultural Safety course.
Active advisories:
Sulfamethoxazole/trimethoprim tablets; peginterferon alfa-2a (Pegasys®) injection; Nicorette 4 mg mint prescription lozenges; oxybutynin oral syrup; calcitrol injection; carbamazepine CR tablets; cholestyramine and colesevelam.
Visit Drug shortages for full list and details.


