Farm inputs - 6.1 Receiving inputs
Damaged, unapproved or wrong inputs received at the farm may cause contamination of the equipment, facility, environment, animals or the food.
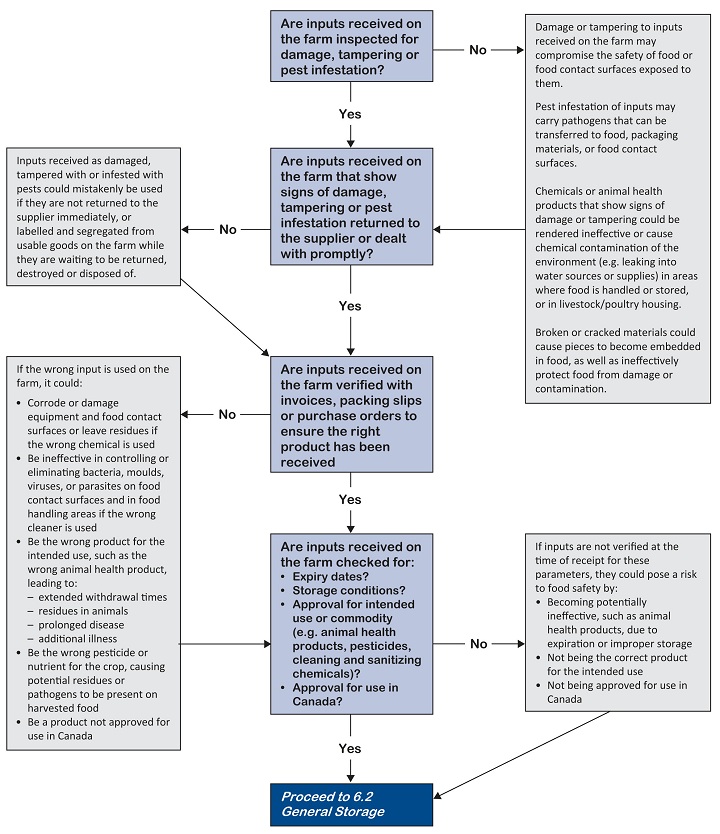
This good agricultural practice applies to all farms.
Examples of inputs are:
|
|
What needs to be done
Receive inputs as ordered, assess them for evidence of food safety hazards (for example, damage, pest infestation, tampering). Where necessary, make sure they are authorized for an intended commodity use in Canada.
How to do it
Consider providing suppliers with a site map of the farm so that inputs are delivered to the desired area.
Perform a visual inspection upon receipt to ensure received inputs:
- Are those ordered
- Are from the intended supplier
- Have intact product seals and have no evidence of contamination, tampering, spoilage, deterioration or damage
- Have a product registration number (e.g. label bearing a registration number or drug identification number [DIN]), if necessary, for the intended purpose and specific for that commodity
- Have not passed their expiry date or are likely to be used up prior to the expiry date
- Are at the appropriate temperature (if applicable)
In addition:
- Sign and date invoices, the bill of lading or the packing slip so that inputs are verified at time of receipt.
- Keep all product inserts as a reference for proper storage and use requirements.
- Ideally, do not accept or unload damaged or rejected materials. In the event that unacceptable material is received, label the item and/or segregate it to prevent contamination until it can be returned to supplier or disposed of by a method that will not cause a food safety hazard. For more information, refer to 6.4 Storage and disposal of farm wastes
- Make sure all received feed is approved for use in livestock and poultry. Consult your feed supplier for more details.
Records to keep
Keep the following records:
- Receipts
- Invoices
- Bill of lading and/or packing slip
Chemical inventory containing name of product received, date received and quantity (and, if necessary, PCP/DIN# and expiry date) records are optional.
If you have an audit
Be prepared for the auditor to review your receipts, invoices, bills of lading, packing slip records or inventory lists.
Laws and regulations
All inputs, including pesticides and animal health products, received on-farm should be approved under various federal and provincial laws and not be prohibited under these laws or regulations made under them (e.g. control products within the Food and Drugs Act (Canada), R.S. 1985, c. F-27, Feeds Act (Canada), R.S. 1985, c. F-9, Fertilizers Act (Canada), R.S. 1985, c. F-10, Hazardous Products Act (Canada), R.S. 1985, c. H-3).
The Veterinary Drug and Medicated Feed Regulation, Reg. 47/82 under the Veterinary Drugs Act regulates the sale of veterinary drugs and describes requirements for recordkeeping, storage, labelling and other measures. Producers may purchase and dispense veterinary drugs and medicated feeds that are permitted to be sold without a prescription as long as they are used according to the label instructions.
| Proceed to 6.2 General storage |
