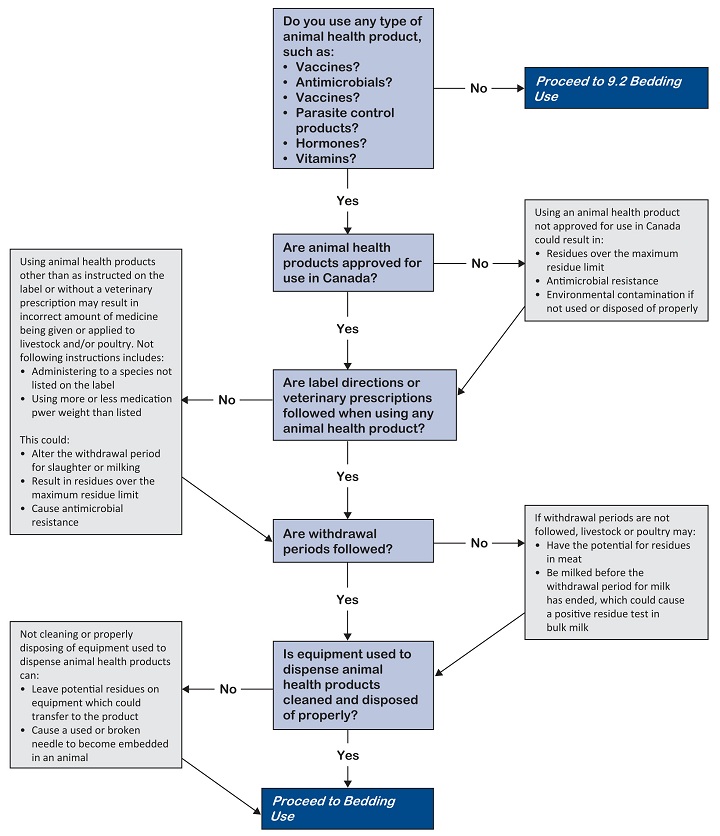
Livestock and poultry - 9.1 Animal health product use
Improper use of animal health products can:
- Reduce product effectiveness
- Cause harmful side effects in animals
- Cause physical contamination (broken needles in meat, water or environmental contamination)
- Possibly lead to antimicrobial resistance
- Result in drug residues in meat, milk, eggs, honey or other products of animal origin
This good agricultural practice applies to all farms using animal health products.
Some examples of animal health products are:
- Alternative health products
- Antimicrobials
- Hormones
- Medicated feed
- Parasite control products
- Vaccines
- Vitamins
- Water-soluble medicines
What needs to be done
Use animal health products properly to avoid excessive residues and broken needles.
How to do it
Develop practices for animal health product use. Written practices include:
- Choosing the right animal health product
- Proper product use and handling
- Use of equipment to apply or administer animal health products
Choose the right animal health product
- Establish a valid client-patient-veterinary relationship with your veterinarian.
- Seek veterinary advice when selecting animal health products to reduce risks of ineffective treatment, drug residues, antimicrobial resistance and environmental contamination.
- Make sure animal health products are approved for use in the species and for the condition being treated.
- When an approved animal health product is not available for a treatment of a species, let your veterinarian select a product to use.
- Obtain a valid veterinary prescription if animal health products or medicated feeds are to be used in an extra-label manner.
Use and handle products properly
Use all animal health products according to label and package insert instructions or according to the direction of your veterinarian. Read and follow the label instructions such as dosage, animal species, and route of administration.
Do not use expired animal health products. Dispose of outdated or unused animal health products, medicated feeds and administration equipment (for example, needles and syringes) in a manner that does not pose a food safety hazard.
Learn about storage and disposal of farm wastes.
Make sure you measure accurately, so that correct concentrations and dosages are achieved.
Store and mix animal health products in clean, correctly labelled containers.
See storage of potentially hazardous products.
Follow proper withdrawal periods to avoid drug residues in milk, eggs, honey, and meat.
Use equipment for animal health products properly
Clean and flush equipment used to dispense, apply or mix an animal health product between uses to avoid cross-contamination of medications or unnecessary injury to animals.
Handle all animals with care, so that animal health products are effectively delivered.
Use needles of appropriate size, based on animal age and weight, route of product delivery and viscosity of product. Consult your veterinarian if unsure. Discard contaminated, dirty, bent or burred needles safely.
If mixing medicated feed on-farm, use sequencing or flushing practices to prevent contamination between mixes.
Operate and maintain water medicators and medicated feed mixing equipment according to manufacturer’s directions.
Learn more about maintenance and calibration and see the Canadian Food Inspection Agency (CFIA) requirements for livestock feed.
Terms used
Approved animal health product is a health product that has passed a Health Canada regulatory process and can be legally sold in Canada. Animal health products are available as non-prescription items (over the counter) or by veterinary prescription only.
Drug residues is the amount of animal health product that may be found in meat, eggs, milk, honey or fish at the point of sale. Health Canada determines what limits are considered safe. Residues below the defined maximum limit are legal; residues above the defined maximum limit are illegal and the lot cannot be sold for human consumption.
Extra-label or off-label use is the use or intended use of a drug approved by Health Canada in an animal in a manner not in accordance with the label or package insert. It also includes the use of all unapproved drugs, including unapproved bulk active pharmaceutical ingredients (APIs) and compounded drugs.
Withdrawal period is the recommended minimum time between the last use of an animal health product and the slaughter of an animal for food or the harvesting of milk or eggs from treated animals or birds for human consumption.
Records to keep
- written practices for animal health product use
- all veterinary prescriptions and product inserts
- Animal Health Product Use Record or your own record that includes:
- Date applied
- Animal, pen, or flock identification
- Reason for application/treatment
- Name of product used, including DIN
- Application or treatment method, including injection location
- Amount applied and/or dosage used (may include weight of animal)
- Withdrawal period required or date safe for slaughter or milking
- Treatment failures, for example broken needles
- Initials of the person who gave the treatment
- Appropriate records for any medicated feeds that are mixed on-farm
If you have an audit
Be prepared for the auditor to review:
- Written practices for animal health product use
- All veterinary prescriptions
- Product inserts
- Animal health product use records
Laws and regulations
There are a number of laws that directly impact on food safety regulating the use of animal health products in agricultural production. However, this section refers only to the use of animal health products that may be transferred to humans through food consumption. It does not include any laws or regulations relating to the use of products to control rabies, West Nile Virus or other such diseases that cannot be spread through eating the food made from the animal or plant.
All animal health products used on-farm must be authorized for agricultural production, approved under various federal and provincial laws and regulations, and sold and used in accordance with these laws. They include the Food and Drugs Act (Canada) R.S. 1985, c. F-27, Food and Drug Regulation, Division 15; Pest Control Products Act (Canada), 2002, c. 28; and requirements of the Integrated Pest Management Act, S.B.C. 2003, c. 58 and Integrated Pest Management Regulation, Reg. 604/04; Feeds Act (Canada), R.S. 1985, c. F-9; and the Hazardous Products Act (Canada), R.S. 1985, c. H-3.
Pesticides, food additives, veterinary drugs, vitamins, mineral nutrients and amino acids must not cause contamination of foods as listed in the Food and Drugs Act (Canada), R.S. 1985, c. F-27, Food and Drug Regulations, Division 15. Under the Food Safety Act, S.B.C. 2002, c. 28, Meat Inspection Regulation, Reg. 349/04, food animals are inspected against the standards related to food safety and animal health established under the Food and Drugs Act (Canada) and the Meat Inspection Act (Canada).
The Veterinary Drugs Act, R.S.B.C. 1996, c. 363, Veterinary Drug and Medicated Feed Regulation, Reg. 47/82, s. 12 (a) requires licensed dispenser to draw the purchaser’s attention to the label information, especially the toxicity warnings and precautions to be taken when using any animal health product (veterinary drug) in animals and poultry intended for human consumption.
The Veterinarians Act, S.B.C. 2010, c. 15, s. 46 (3) provides that farmers or their household members or agricultural employees do not need to be a licensed Veterinarian in order to treat their own animals. However, off-label use requires veterinary advice or other legal approval. Veterinarians who prescribe off-label use must advise recipients of appropriate withholding times (during which the animal cannot be sold for consumption).